Innovative Artificial Cornea Transplant at Price Vision Group
Corneal Transplants
A corneal transplant involves replacing a cloudy cornea with a clear donor cornea. Donor tissue comes from individuals who have donated their eyes for the benefit of others. The donor corneas used in transplants are kept freshly stored in eye banks. All of the corneas come from eye banks in the United States, which are regulated by the FDA. The success rate of corneal transplants is amazingly good, nearly 95%. One reason for that amazing statistic is that human corneal tissue is one of the few tissues which can be transplanted with very little risk of rejection. Newer transplantation techniques are showing promise of the rejection rate becoming even lower.
Posterior Lamellar Cornea Transplants
Corneal conditions that affect the inner layer of the cornea are treated with a posterior lamellar corneal transplant. Replacing just the posterior part of the cornea has many advantages over the traditional full thickness graft. The visual recovery after a posterior graft is much more rapid. Normally only one or two sutures are inserted and these can be removed in a week post operatively compared to 16 sutures for a full thickness graft which remain for at least a year. The eye is stronger after this type of transplant and less prone to accidental injury. The refractive end point after a posterior graft is also more predictable and patients can typically see well with just glasses.
Artificial Cornea Transplant
It has been estimated that up to 10 million people in the world suffer corneal blindness. Corneal blindness exists when the rest of the eye functions fully, and the cornea is the only part of the eye needed to restore sight. It may be caused by and accident or from various diseases that damage the front of the eye.
 Many people with corneal disease can be helped by regular corneal transplantation using tissue transplanted from human donors, however, in some cases such transplantation is hopeless.
Many people with corneal disease can be helped by regular corneal transplantation using tissue transplanted from human donors, however, in some cases such transplantation is hopeless.
Currently there are only 100,000 corneal transplants performed each year worldwide due to the lack of access to donor tissue, which has been described as an international crisis in public health. Additionally, many of these donor transplants are rejected by the host body or simply do not work.
After more than 200 years of research the artificial cornea was developed. Indianapolis ophthalmologist and founder of the Cornea Research Foundation of America Dr. Francis W. Price, Jr of the Price Vision Group successfully performed the first artificial cornea transplant, the AlphaCor, in Indiana in 2004.
The surgeons of Price Vision Group now use the Boston K-Pro artificial cornea. It is the most widely used artificial cornea or keratoprosthesis. The K-Pro is a treatment option for corneal disease not amendable to standard penetrating keratoplasty (PKP) or corneal transplant. Continued advances in design and superior postoperative care have resulted in improved outcomes.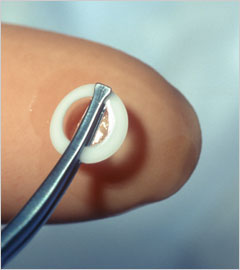
The Boston K-Pro keratoprosthesis is made of medical-grade polymethylmethacrylate (PMMA) the same material that is used in some contact lenses and intraocular lens implants. The device snaps together with corneal graft tissue sandwiched in-between and is then sutured in to replace the cloudy cornea. A soft contact lens is applied following surgery as a bandage and is required full time following surgery-for life.
Indications
The Boston K-Pro has been used in patients with multiple graft failures, Stevens-Johnson syndrome, ocular cicatricial pemphigoid, other autoimmune diseases, ocular burns, aniridia and other conditions with poor prognosis with a traditional corneal transplant.
Risks and Discomforts
The prognosis following a Boston K-Pro is very good. The majority of patients do very well and vision as a rule becomes much improved.
Complications can occur. Necrosis and melting of the tissue around the keratoprosthesis, although rare, can occur. Infections are also very rare as long as prophylactic antibiotic eye drops are instilled as directed – for life.
Eyes needing a keratoprosthesis often have glaucoma requiring anti-glaucoma medications, and sometimes glaucoma surgery is indicated to control glaucoma in conjunction with the K-Pro. It is more difficult to accurately measure the intraocular pressure following a keratoprosthesis, so more frequent visual fields and ocular nerve evaluations are required.
In more severely inflamed eyes, a membrane can form behind the keratoprosthesis. Usually, this can be opened up with laser treatment if needed.
Because of these possible complications, which can result in the functional loss of the eye, patients with keratoprosthesis require regular ophthalmological examination. It is customary to return every month for a check during the first 6 months following surgery and then every 3 months for life. Medications will have to be taken for life. Also, a bandage soft contact lens must be worn around the clock. The K-Pro patient must be available for follow-ups on a regular basis.
For more information on this revolutionary procedure, please contact Price Vision Group at (317) 844-5530

